Atmosphere emission sources includes oxygen molecules, carbon dioxide, water vapor, droplets of water, dust aerosols and sulfuric acid aerosols (polar stratospheric cloud droplets). At frequencies where the MTP operates, and at the high altitudes where the MTP is usually flown, the only emission source to consider is oxygen molecules. Flight at lower altitudes, where clouds are present, would have to include liquid water droplets.
Oxygen
The MTP operates at frequencies where oxygen molecules interact with microwaves. Oxygen molecules rotate with energies that differ by amounts that correspond to the energy of microwave photons in the frequency range 48 to 71 GHz (and at 119 GHz). At sea level the typical oxygen molecule is distorted so much from recent collisions that a molecule's change in energy as it switches from one rotational state to another can differ from the low pressure energy difference, and this causes a "pressure broadening" of the resonance frequency. As a result of pressure broadening the oxygen molecule absorption spectrum has a structure that variers significantly with altitude, as the following graph illustrates.
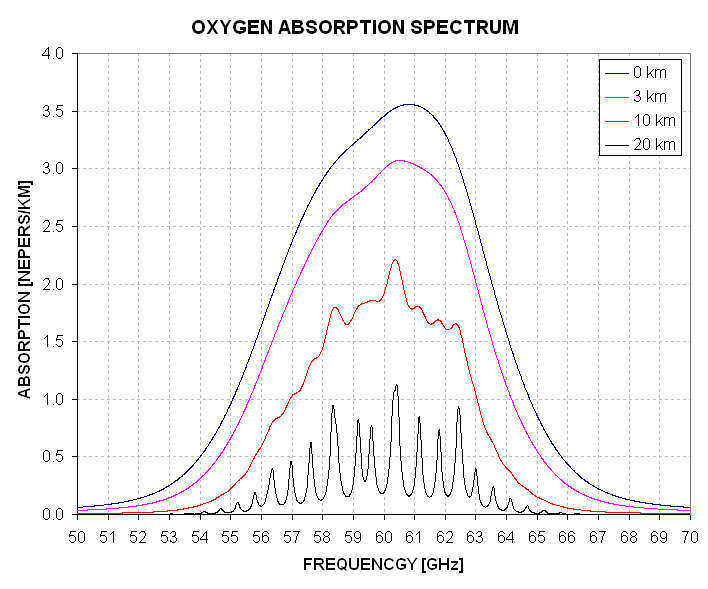
Figure 5.1 Oxygen molecule absorption spectrum for 4 altitudes (using US Standard Atmosphere temperatures and pressures).
Absorption coefficient, Kv, can be given in units of Nepers per kilometer [Neper/km]. To convert to [db/km] multiply the [Neper/km] value by 4.343. In a medium where Kv = 1 [Neper/km] a photon will have a 1/e = 0.37 probability of being transmitted 1 km (i.e., without being absorbed). It will also have a 1 - 1/e = 0.73 probability of being absorbed. Every medium that absorbs photons also emits them, and oxygen molecules are the main source of this thermal radiation at frequencies near 60 GHz.
Carbon Dioxide
Carbon dioxide molecules don't interact with photons having frequencies below 115 GHz, so they can be ignored for the MTP.
Water Vapor
Water vapor molecules have a resonate absorption features at 22.2 GHz and 183 GHz, as well as a poorly understood non-resonant component at microwave frequencies. Water vapor has a distribution versus altitude characterized by a much smaller scale height than dry air. If relative humidity were constant with altitude then water vapor would have a scale height of ~2 km instead of the dry atmosphere's 8 km, and in fact this 2 km scale height for water vapor is indeed found to hold quite often. Because most water vapor molecules are found near the surface their presence at typical MTP altitudes can be ignored. For those few occasions that an MTP is operated within the planetary boundary layer it is still acceptable to ignore water vapor absorption since at these low altitudes the level of oxygen molecule absorption is still much higher. Nevertheless, here is the water vapor absorption spectrum near sea level.
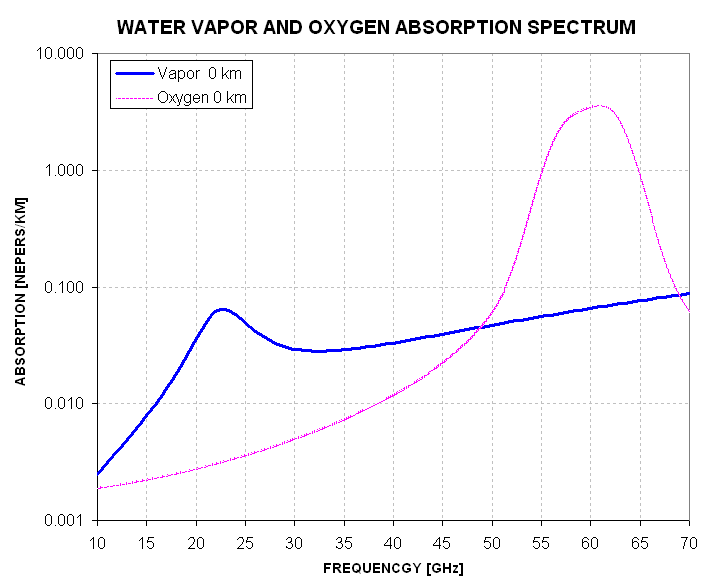
Figure 5.2 Water vapor absorption for typical sea level conditions in relation to oxygen absorption. An MTP operates at frequencies in the 55 to 60 GHz region.
Considering that the MTP operates at frequencies near the peak for oxygen absorption (55 to 60 GHz) when an MTP flies at low altitudes the additional absorption of the atmosphere due to water vapor is small in relation to that of oxygen molecules, being approximately 50 times smaller. At normal flight altitudes the water vapor contribution is even smaller due to the difference in scale heights for water vapor and oxygen.
The semi-empirical equation for water vapor absorption is given by Waters (1976):
Kv [Neper/km] = V * W * F2 * T -3/2 * { (7.18 * 10 5 e-644/T ) / (T * X) + 2.77 * 10 -3 }
where W [GHz] = 2.46 * (P / 1013) * ( 300 / T ) 0.626 * ( 1 + 0.018 * ( V * T ) / P )
and X [GHz2] = ( 494.4019 - F 2 ) + ( 2 * F * W )
V [gm / m3] = water vapor density
P [mb] = air pressure
T [K] = air temperature
F [GHz] = frequency
Notice that there's a "resonance" feature at SQRT(494.4019) = 22.235 [GHz]. The "line width parameter" W accomodates the fact that high pressure broadens the resonant absorption feature, which causes absorption at line center to decrease while farther than ~1.63 GHz from line center absorption increases. At MTP frequencies (53 to 60 GHz) colder means more absorptive, and lower pressure means lower absorption. However, changes in altitude correspond to water vapor mixing ratio changes that are more important than pressure or temperature changes, as the following graph shows.
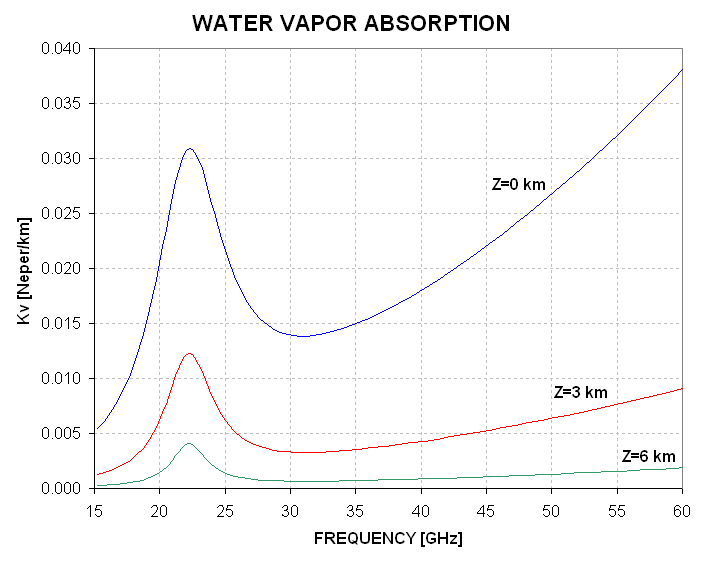
Figure 5.3 Water vapor absorption for typical conditions at 3 altitudes (1000, 700 and 300 mb).
Since water vapor mixing ratio (and density) decreases with a scale height of about 2 km small increases in altitude correspond to large decreases in water vapor absorption. At tropospheric altitudes (10 to 16 km) water vapor absorption can be ignored.
Cloud Liquid Water Droplets
Most cloud liquid water drops are small compared to microwave wavelengths. Only large rain drops are comparable in circumference to wavelength, which means that cloud drops absorb in a way that is not influenced by drop size. This is good, because it means that clouds absorb in proportion to their average volume liquid water content, expressed in units of grams per cubic meter. The term "liquid water content" (LWC) is commonly used. A typical cloud near the surface has LWC = 0.5 [g/m3]. Here's the absorption spectrum for such a cloud.
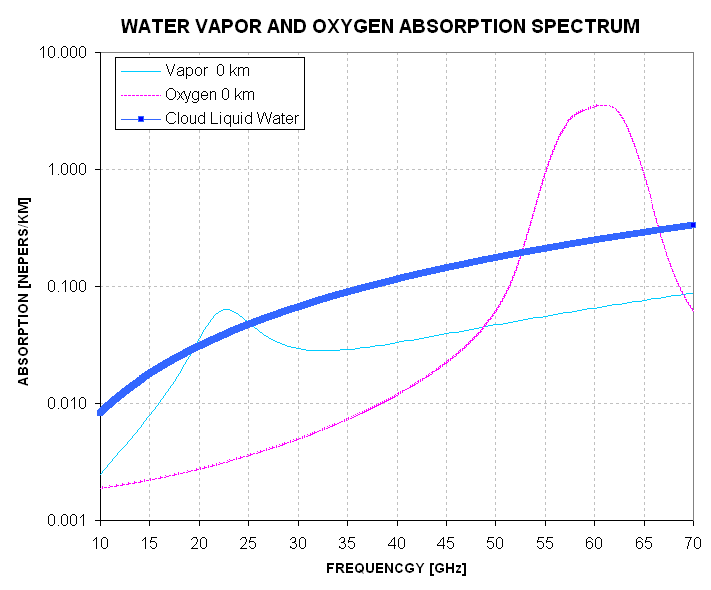
Figure 5.4 Liquid water (cloud) absorption for typical sea level conditions in relation to oxygen absorption. The cloud is assumed to have LWC = 0.5 [g/m3] and a temperature of 288 K.
This graph shows that within a typical cloud layer (at low altitude) the liquid water droplets absorb more MTP photons per unit distance than water vapor, though less than oxygen. Keep in mind that this compononet of absorption is confined to the cloud layer (which might be 1 km) whereas the other two components extend over a much larger altitude region. For an MTP flying in the boundary layer absorption by oxygen will exceed that for any other component by 1 to 2 orders of magnitude.
Cumulus clouds have low LWC values, typically 0.1 [g/m3], which produce an absorption spectrum 4 times lower than in the above graph. Only stratus clouds have large LWC values, approaching 1.0 [g/m3].
Clouds that are colder than freezing are either a mixture of water and ice (in the temperature region 0 C to -40 C) or pure ice crystals (colder than -40 C). Pure ice crystals are nearly transparent at MTP frequencies. A typical cirrus cloud consisting of only ice crystals would have to be ~400 km thick to produce one Neper of absorption at 60 GHz. Therefore, cirrus clouds (most of which are pure ice crystals) can be ignored by MTP users.
The equation for liquid water absorption is:
Kv [Np/km] = 0.075 * LWC [g/m3] * (280 / T[K]) 6.87 * (F / 22.2 [GHz]) 1.9
where F is frequencey [GHz]. This equation shows that cold clouds are more absorptive than warm ones. For every 10 K of additional coldness the liquid water absorption increases ~28%. Note the exponent for the frequency dependence, 1.9. This exponent would be 2.0 if cloud droplets were all much smaller than wavelength. Since some droplets are comparable to wavelength, the 1.9 exponent provides a better empirical fit to real-world clouds. Cloud droplets are poor scatterers at MTP wavelengths (because of their small size), and this component of "loss" can be ignored. Rain drops can scatter at the few tens of percent of ozygen absorption at 60 GHz. Even for these conditions liquid water absorption is more important than scattering.
Aerosols
Non-water aerosols consist of dust and smog (at low altitudes), which are unimportant at MTP frequenceis. For example, a dust storm with an optical scattering loss having an e-folding distance of 500 meters will produce a only a 0.3 % loss per 500 meters at MTP frequencies (assuming 100 micron diameter aerosols). Oxygen absorption is 2 to 3 orders of magnitude greater. Smog does not have absorption features at microwave frequenceis (the important ones are at higher frequencies).
At high altitudes (in the lower stratospehre) the only aerosols that can possibly affect MTP absorption are small droplets of sulfuric acid, liquid water and nitric acid (polar stratospheric). The Type I PSC aerosols consist of nitric acid tri-hydrate (NAT) particles ~1 or 2 microns in diameter. They are found where temperatures are colder than 195 K and where N2O mixing ratios are high (i.e., above the tropopause). When temperatures drop below 190 K water vapor swells the NAT aerosols, producing Type II PSCs. These aerosols have diamters of 5 to 20 microns (the larger ones have fall rates that "de-nitrify" the layer with large Type II aerosols). I have calculated that Type I and Type II PSC aerosols are not detectable through their absorption effects at 60 GHz. Scattering by these aerosols is also too small for detection. Volcanic clouds have two components, ash (dust) and sulfuric acid, and typically they will be together as a droplet of sulfuric acid with an ash grain inside. These aerosols produce negligible absorption and scattering compared with that of oxygen molecules. Condensation nuclei are all too small to affect microwaves.
This concludes a survey of potential atmospheric absorbers at MTP frequencies. The "message" presented is a reassuring one, namely, only oxygen molecular absorption needs to be considered for an MTP flying above the planetary boundary layer. The next chapter makes this assumption as we begin to explore ways to retrieve temperature profiles from MTP observables.
Go to Chapter #6 (next chapter)
This is Chapter 5
Go to Chapter #4 (previous chapter)
Return to Introduction
____________________________________________________________________